- Clinical Experience
- Rheumatology
- Pulmonology
- Ophthalmology
- Nephrology
- Neurology
- Proposed MOA
- Dosing & Administration
- Safety Data
- Support & Resources
-
Clinical Studies
OPEN-LABEL STUDY AGGARWAL FOLLOW-UP STUDY PROSPECTIVE STUDY RETROSPECTIVE STUDY
Case Studies
PM CLINICAL CASE STUDY DM CLINICAL CASE STUDY Aggarwal R, Marder G, Koontz DC, Nandkumar P, Qi Z, Oddis CV—Annals of the Rheumatic Diseases, 2018
Disclosure statement: Funding to support this study was provided by Mallinckrodt Pharmaceuticals.
An open-label, 24-week study of Acthar® Gel used in 11 adults with refractory and active DM/PM who needed an alternative to glucocorticoids and/or conventional immunosuppressive agents1
Objective
To evaluate the efficacy, safety, tolerability, and glucocorticoid-sparing effect of Acthar Gel in an open-label, 24-week, proof-of-concept study of 11 adults with refractory and active DM/PM*
*Ten of the 11 enrolled patients completed the study. One patient dropped out due to heart block unrelated to the study drug and was not included in the efficacy analysis, as she did not complete the minimum 8 weeks of the study drug required for outcome assessment per study protocol.
Study Design1
- A 24-week proof-of-concept study of 11 adults with refractory and active DM/PM*
- Refractory disease defined as failing an adequate glucocorticoid trial (≥2 months of high doses [0.75 mg/kg to 1 mg/kg] or intolerance to such therapy) and/or ≥1 conventional immunosuppressive agent
- Active disease defined as having:
- Baseline Manual Muscle Testing (MMT-8) score ≤125 out of 150 and ≥2 additional abnormal core set measures (CSMs), or
- DM with cutaneous 10-cm visual analog scale (VAS) score ≥3 cm on the Myositis Disease Activity Assessment Tool (MDAAT) and at least 3 other abnormal CSMs (excluding the MMT-8)
- All patients received 80 units of Acthar Gel twice weekly for 24 weeks†
Study Assessments1
Primary endpoint
- Three of any of the 6 CSMs improved by ≥20%, with no more than 2 CSMs worsening by ≥25%, based on IMACS definition of improvement (DOI)
- Worsening measure could not include MMT
- Primary endpoint was also separately evaluated on a subset of patients with severe muscle weakness (defined as an MMT score of ≤125 out of 150 at baseline), as well as moderate to severe cutaneous DM rashes (defined as a cutaneous VAS score ≥2.5 out of 10 at baseline)
Secondary endpoints
- Median change in 6 individual CSMs
- Median time to definition of improvement (DOI) from baseline
- 2016 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) myositis response criteria
- Mean change in glucocorticoid dose at 24 weeks
- Secondary safety and tolerability endpoints were measured by frequency and type of adverse and severe adverse events (AEs and SAEs)
AE=adverse event; IMACS=International Myositis Assessment and Clinical Studies Group; SAE=serious adverse event.
†Concomitant immunosuppressive agents or glucocorticoids were allowed as long as patients were on these therapies for ≥8 weeks (and ≥4 weeks for glucocorticoids) and on a stable dose for ≥4 weeks and ≥2 weeks, respectively, prior to the start of the trial.
Patient Characteristics1
DM/PM patients had chronic and persistent disease activity and were previously treated with other regimens1
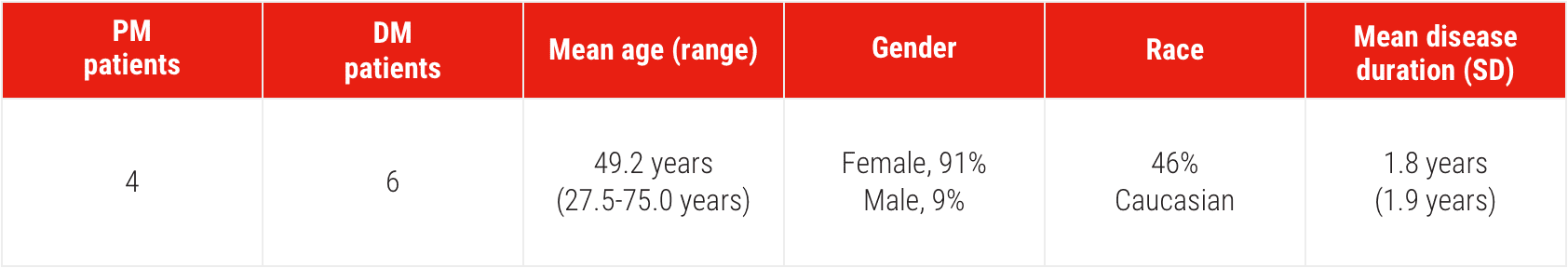
SD=standard deviation.
Study Limitations1
- Efficacy results are based on 10 patients who completed the study
- This study may not be fully representative of outcomes in the overall patient population
- Sample bias may exist, as this was an open-label study
- All patients were on multiple therapies; therefore, the clinical outcomes may not be solely attributable to Acthar Gel
- Acthar Gel has not been formally studied in combination with other treatments
70% of patients (n=7) met the primary endpoint after treatment with Acthar Gel1
Primary Endpoint1
- Seven out of 10 patients completing the study met the IMACS DOI by a median of 8 weeks (interquartile range=4 to 20 weeks) after treatment with Acthar Gel
- DM and PM patients did not differ in their response to treatment
Primary endpoint: definition of improvement
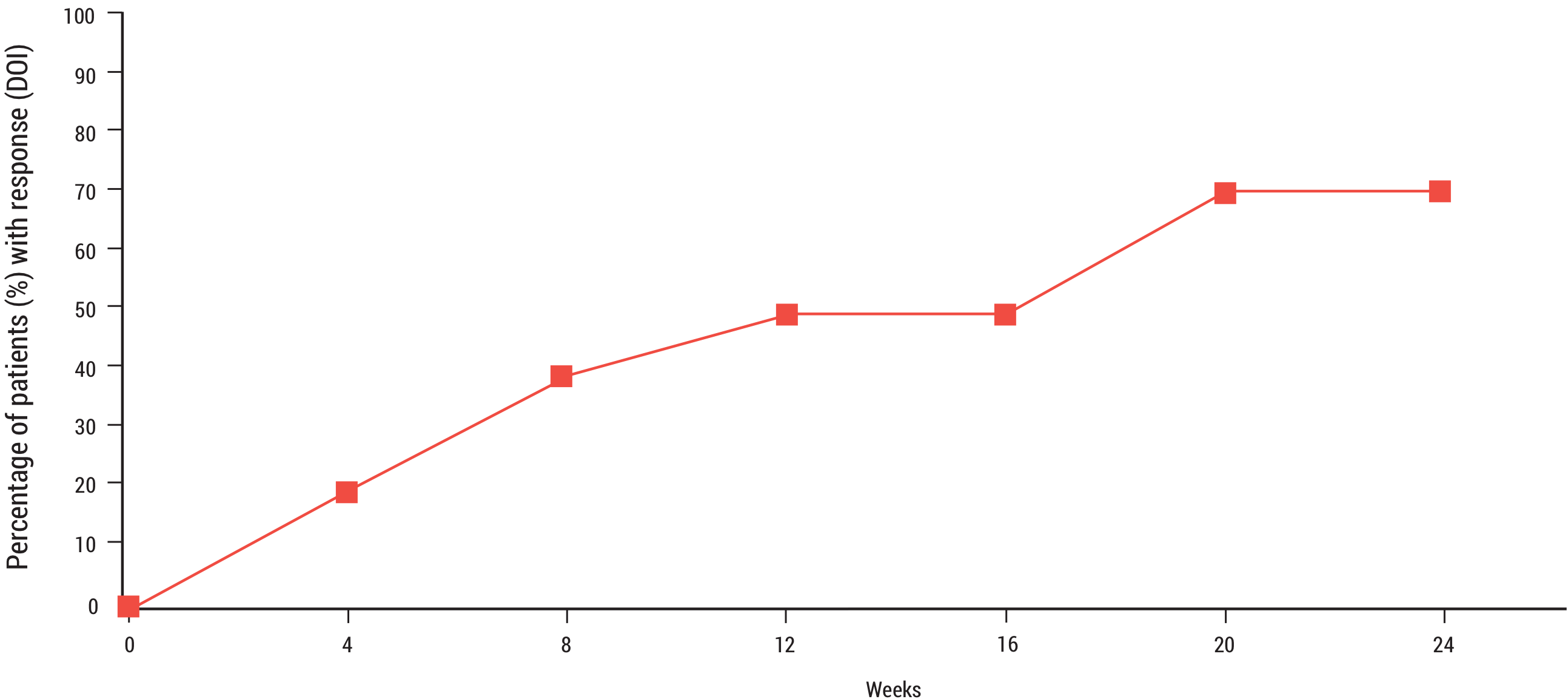
DOI=definition of improvement; IMACS=International Myositis Assessment and Clinical Studies Group.
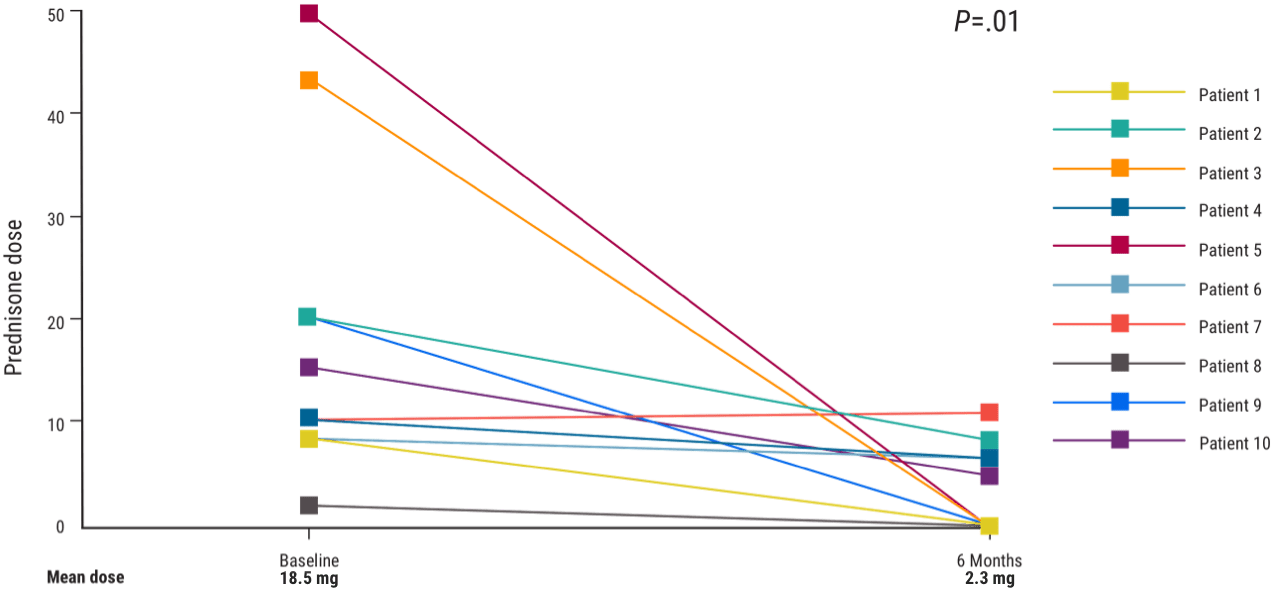
KEY Secondary Endpoint1
All patients (n=10)* decreased or discontinued prednisone doses by Week 241
50% (n=5) of patients discontinued prednisone completely by Week 24
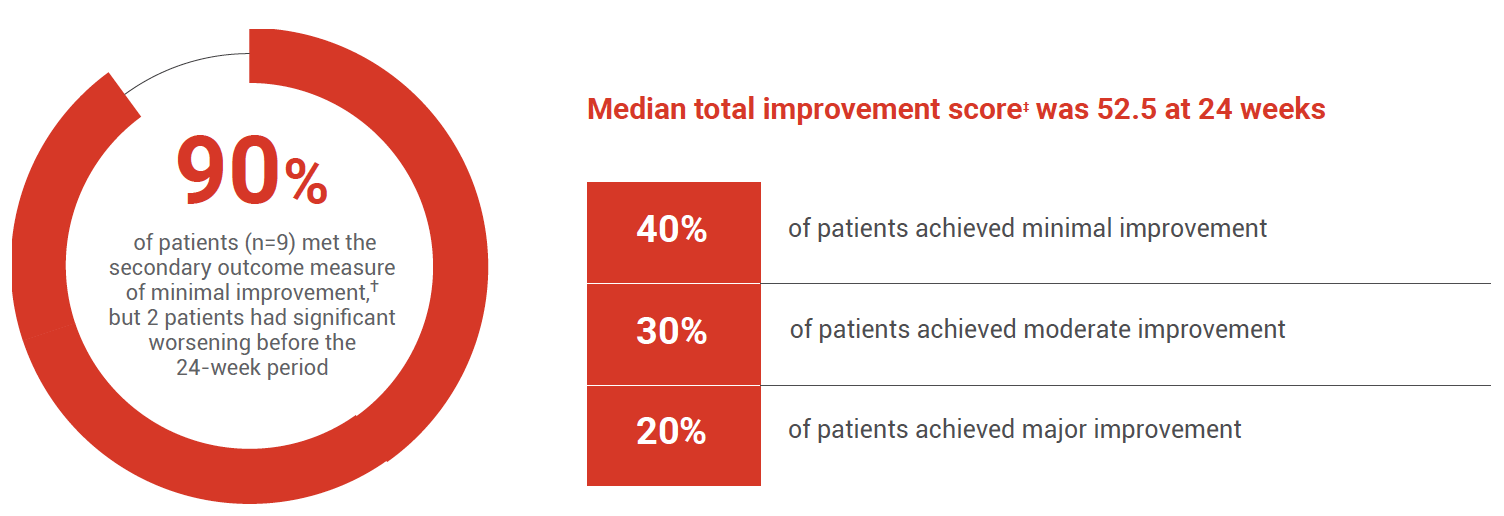
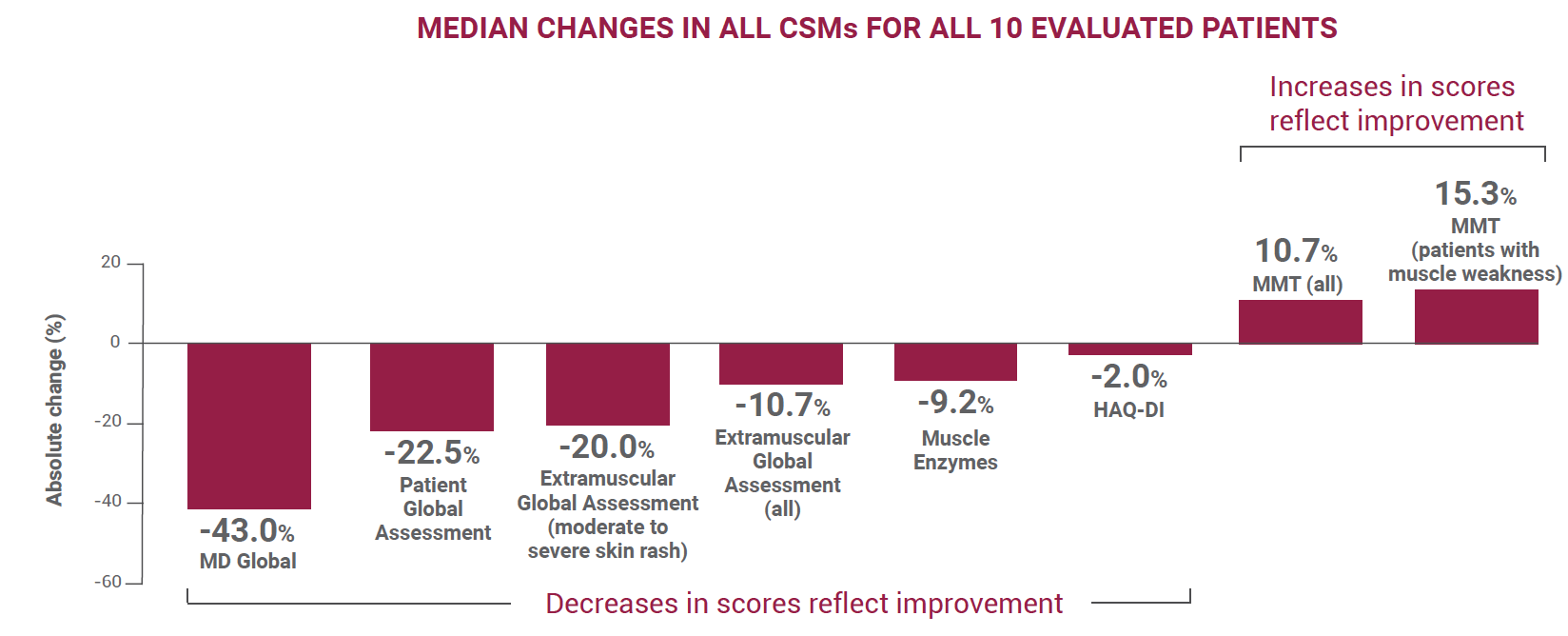
Patients showed improvements in several CSMs
- MMT improved by 10.7% in all patients
- Seven patients with severe muscle weakness at baseline showed median improvement of 15.3%
- MD Global improved by >43% in all 10 patients
- Extramuscular Global Assessment improved by 10.7% in all patients
- Patients with moderate to severe rash at baseline showed improvement of 20%
Results are based on 10 patients who completed the study. This study may not be fully representative of outcomes in the overall patient population. All patients were on multiple therapies; therefore, the clinical outcomes may not be solely attributable to Acthar Gel. Acthar Gel has not been formally studied in combination with other treatments.
*Ten of the 11 enrolled patients completed the study.
†Criteria for improvement were based on the 2016 ACR/EULAR myositis response criteria.
‡Metric derived from the 2016 ACR/EULAR myositis response criteria, which corresponds to magnitude of improvement.
Safety ENDPOINTS1
Many of the observed AEs were similar to those seen with glucocorticoids. Other factors typically associated with high-dose steroids given for an extended period, such as significant weight gain, diabetes, or Cushingoid features, were not observed.
- A total of 5 SAEs were observed in 3 patients
- Of these SAEs, 3 were related to the study drug
- Two patients experienced herpes zoster infections and required hospitalization, including 1 patient with disseminated herpes zoster causing herpes pneumonitis
- A total of 22 AEs were observed in 8 patients, all related to the study drug
SUMMARY OF ADVERSE EVENTS
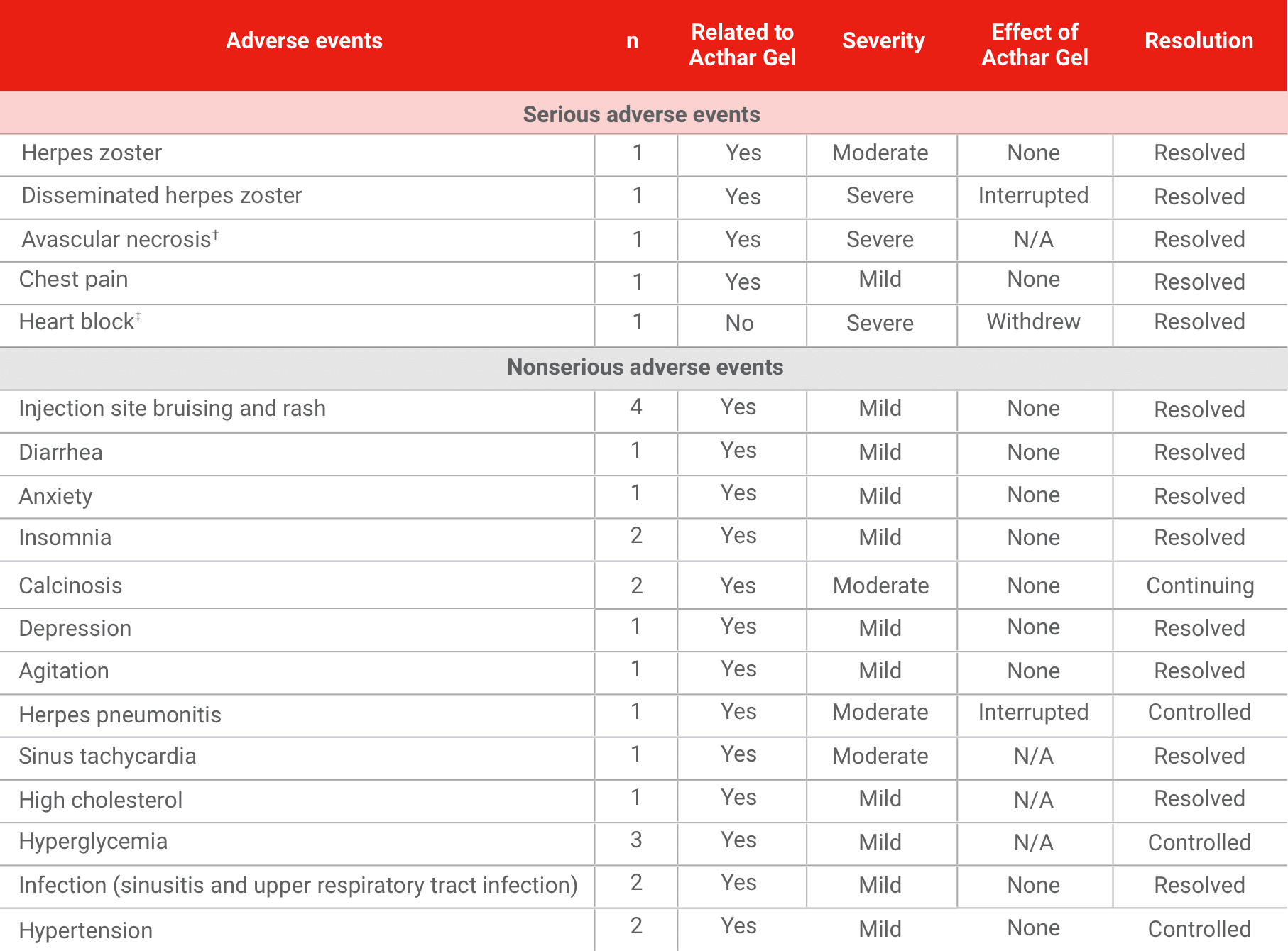
Results are based on 10 patients who completed the study. This study may not be fully representative of outcomes in the overall patient population. All patients were on multiple therapies; therefore, the clinical outcomes may not be solely attributable to Acthar Gel. Acthar Gel has not been formally studied in combination with other treatments.
AE=adverse event; N/A=not available; SAE=serious adverse event.
†Total left hip arthroplasty.
‡Transvenous pacemaker insertion.

Prescribe Acthar Gel now
Start the referral process for your appropriate patients

Dosing recommendations
See additional dosing information from clinical experience with Acthar Gel
INDICATIONS
Acthar Gel is indicated for:
- Treatment during an exacerbation or as maintenance therapy in selected cases of systemic dermatomyositis (polymyositis)
- Treatment during an exacerbation or as maintenance therapy in selected cases of systemic lupus erythematosus
- Adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in: psoriatic arthritis; rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy); ankylosing spondylitis
- Symptomatic sarcoidosis
- Severe acute and chronic allergic and inflammatory processes involving the eye and its adnexa such as: keratitis, iritis, iridocyclitis, diffuse posterior uveitis and choroiditis, optic neuritis, chorioretinitis, anterior segment inflammation
- Inducing a diuresis or a remission of proteinuria in nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosus
- Treatment of acute exacerbations of multiple sclerosis in adults. Controlled clinical trials have shown Acthar to be effective in speeding the resolution of acute exacerbations of multiple sclerosis. However, there is no evidence that it affects the ultimate outcome or natural history of the disease
- Monotherapy for the treatment of infantile spasms in infants and children under 2 years of age
Important Safety information
Contraindications
Acthar is contraindicated:
- For intravenous administration
- In infants under 2 years of age who have suspected congenital infections
- With concomitant administration of live or live attenuated vaccines in patients receiving immunosuppressive doses of Acthar
- In patients with scleroderma, osteoporosis, systemic fungal infections, ocular herpes simplex, recent surgery, history of or the presence of a peptic ulcer, congestive heart failure, uncontrolled hypertension, primary adrenocortical insufficiency, adrenocortical hyperfunction, or sensitivity to proteins of porcine origin
Warnings and Precautions
- The adverse effects of Acthar are related primarily to its steroidogenic effects
- Acthar may increase susceptibility to new infection or reactivation of latent infections
- Suppression of the hypothalamic-pituitary-adrenal (HPA) axis may occur following prolonged therapy with the potential for adrenal insufficiency after withdrawal of the medication. Adrenal insufficiency may be minimized by tapering of the dose when discontinuing treatment. During recovery of the adrenal gland patients should be protected from the stress (e.g., trauma or surgery) by the use of corticosteroids. Monitor patients for effects of HPA axis suppression after stopping treatment
- Cushing’s syndrome may occur during therapy but generally resolves after therapy is stopped. Monitor patients for signs and symptoms
- Acthar can cause elevation of blood pressure, salt and water retention, and hypokalemia. Monitor blood pressure and sodium and potassium levels
- Acthar often acts by masking symptoms of other diseases/disorders. Monitor patients carefully during and for a period following discontinuation of therapy
- Acthar can cause gastrointestinal (GI) bleeding and gastric ulcer. There is also an increased risk for perforation in patients with certain GI disorders. Monitor for signs of perforation and bleeding
- Acthar may be associated with central nervous system effects ranging from euphoria, insomnia, irritability, mood swings, personality changes, and severe depression to psychosis. Existing conditions may be aggravated
- Patients with comorbid disease may have that disease worsened. Caution should be used when prescribing Acthar in patients with diabetes and myasthenia gravis
- Prolonged use of Acthar may produce cataracts, glaucoma, and secondary ocular infections. Monitor for signs and symptoms
- Acthar is immunogenic and prolonged administration of Acthar may increase the risk of hypersensitivity reactions. Cases of anaphylaxis have been reported in the postmarketing setting. Neutralizing antibodies with chronic administration may lead to loss of endogenous ACTH and Acthar activity
- There may be an enhanced effect in patients with hypothyroidism and in those with cirrhosis of the liver
- Long-term use may have negative effects on growth and physical development in children. Monitor pediatric patients
- Decrease in bone density may occur. Bone density should be monitored in patients on long-term therapy
Adverse Reactions
- Commonly reported postmarketing adverse reactions for Acthar include injection site reaction, asthenic conditions (including fatigue, malaise, asthenia, and lethargy), fluid retention (including peripheral swelling), insomnia, headache, and blood glucose increased
- The most common adverse reactions for the treatment of infantile spasms (IS) are increased risk of infections, convulsions, hypertension, irritability, and pyrexia. Some patients with IS progress to other forms of seizures; IS sometimes masks these seizures, which may become visible once the clinical spasms from IS resolve
Pregnancy
- Acthar may cause fetal harm when administered to a pregnant woman
Please see full Prescribing Information for additional Important Safety Information.
References:
- Baughman RP, Barney JB, O’Hare L, Lower EE. A retrospective pilot study examining the use of Acthar Gel in sarcoidosis patients. Respir Med. 2016;110:66-72.
- Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313-322.
- Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, Khastgir A. Acthar Gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17:37.
- Aggarwal R, Marder G, Koontz DC, Nandkumar P, Qi Z, Oddis CV. Efficacy and safety of adrenocorticotropic hormone gel in refractory dermatomyositis and polymyositis. Ann Rheum Dis. 2018;77(5):720-727.
- Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. 2014;23(9):905-912.
- Alhamad T, Dieck JM, Younus U, et al. ACTH Gel in resistant focal segmental glomerulosclerosis after kidney transplantation. Transplantation. 2019;103(1):202-209.
- Nelson WW, Lima AF, Kranyak J, et al. Retrospective medical record review to describe use of repository corticotropin injection among patients with uveitis in the United States. J Ocul Pharmacol Ther. 2019;35(3):182-188.
- Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13:1-11
- Zand L, Canetta P, Lafayette R, et al. An open-label pilot study of adrenocorticotrophic hormone in the treatment of lgA nephropathy at high risk of progression. Kidney Int Rep. 2020;5(1):58-65.
- Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327-344.
- Ho-Mahler N, Turner B, Eaddy M, Hanke ML, Nelson WW. Treatment with repository corticotropin injection in patients with rheumatoid arthritis, systemic lupus erythematosus, and dermatomyositis/polymyositis. Open Access Rheumatol. 2020;12:21-28.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Data on File – Ref-06550. Mallinckrodt Pharmaceuticals.
- Data on File – Ref-05336. Mallinckrodt Pharmaceuticals.
- Data on File – Ref-07068. Mallinckrodt Pharmaceuticals.
- Wirta D, Mclaurin E, Ousler G, Liu J, Kacmaz RO, Grieco J. Repository corticotropin injection (Acthar® Gel) for refractory severe noninfectious keratitis: efficacy and safety from a phase 4, multicenter, open-label study. Ophthamol Ther. 2021;10(4):1077-1092.
- Tumlin J, Galphin C, Santos R, Rovin B. Kidney Int Rep. 2017;2(5):924-932.
- Grafals M, Sharfuddin A. Adrenocorticotropic hormone in the treatment of focal segmental glomerulosclerosis following kidney transplantation. Transplant Proc. 2019;51(6):1831-1837.
- Hladunewich MA, Cattran D, Beck LH, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29(8):1570-1577.
- Filippone EJ, Dopson SJ, Rivers DM, et al. Adrenocorticotropic hormone analog use for podocytopathies. Int Med Case Rep J. 2016;9:125-133.
- Bomback AS, Tumlin JA, Baranski J, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011;5:147-153.
- Bomback AS, Canetta PA, Beck LH Jr, Ayalon R, Radhakrishnan J, Appel GB. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36(1):58-67.
- Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11:598496.
- Bryan MS, Sergott RC. Changes in visual acuity and retinal structures following repository corticotropin injection (RCI) therapy in patients with acute demyelinating optic neuritis: improvement in low contrast visual acuity in both affected and contralateral eyes in a single-armed open-label study. J Neurol Sci. 2019;407:116505.
- Mallinckrodt Pharmaceuticals. Acthar® Gel (repository corticotropin injection) Reference Bibliography. https://www.mallinckrodt.com/globalassets/documents/corporate/bibliography/acthar-gel-repository-corticotropin-injection-reference-bibliography.pdf. Accessed December 7, 2023.
- Wynn D, Goldstick L, Bauer W, et al. Results from a multi-center, randomized, double-blind, placebo-controlled study of repository corticotropin injection for multiple sclerosis relapse that did not adequately respond to corticosteroids. CNS Neurosci Ther. 2022;28:364-371.
- Saygin D, Oddis CV, Marder G, et al. Follow-up results of myositis patients treated with Acthar gel. Rheumatology (Oxford). 2020;59(10):2976-2981.
- Fernandez AP, Gallop J, Polly S, Khanna U. Efficacy and safety of repository corticotropin injection for refractory cutaneous dermatomyositis: a prospective, open-label study. Rheumatology (Oxford). 2023 Nov 6. doi: 10.1093/rheumatology/kead595. Epub ahead of print.
- Anesi SD, Chang PY, Maleki A, et al. Treatment of noninfectious retinal vasculitis using subcutaneous repository corticotropin injection. J Ophthalmic Vis Res. 2021;16(2):219-233.
- Anesi SD, Chang PY, Maleki A, et al. Effects of subcutaneous repository corticotropin gel injection on regulatory T cell population in noninfectious retinal vasculitis. Ocul Immunol lnflamm. 2023;31(3):556-565.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Huang JY, Galen K, Zweifel B, Brooks LR, Wright AD. Distinct binding and signaling activity of Acthar Gel compared to other melanocortin receptor agonists. J Recept Signal Transduct Res. 2020;1-9. doi:10.1080/10799893.2020.1818094.
- Healy LM, Jang JH, Lin YH, Rao V, Antel JP, Wright D. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages [ECTRIMS-ACTRIMS abstract EP1481]. Mult Scler J. 2017;23(suppl 3):777.
- Wright D, Hayes K. Acthar Gel inhibits the activation of CD4+ and CD8+ T cells. J Interferon Cytokine Res. 2023;43(4):182-187. doi: 10.1089/jir.2022.0257.
- Olsen NJ, Decker DA, Higgins P, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300. doi:10.1186/s13075-015-0823-y.
- U.S. National Library of Medicine. DailyMed website. https://dailymed.nlm.nih.gov/dailymed/. Accessed March 3, 2022.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Catania A, Lonati C, Sordi A, Carlin A, Leonardi P, Gatti S. The melanocortin system in control of inflammation. ScientificWorldJournal. 2010;10:1840-1853. doi:10.1100/tsw.2010.173.
- Huang YJ, Galen K, Zweifel B, Brooks LR, Wright AD. Distinct binding and signaling activity of Acthar Gel compared to other melanocortin receptor agonists. J Recept Signal Transduct Res. 2021;41(5):425-433.
- Olsen NJ, Decker DA, Higgins P, et al. Direct effects of HP Acthar Gel on human B lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300. doi:10.1186/s13075-015-0823-y.
- Healy LM, Jang JH, Lin YH, Rao V, Antel JP, Wright D. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages [ECTRIMS-ACTRIMS abstract EP1481]. Mult Scler J. 2017;23(suppl 3):777.
- Wright D, Hayes K. Acthar Gel inhibits the activation of CD4+ and CD8+ T cells. J Interferon Cytokine Res. 2023;43(4):182-187. doi: 10.1089/jir.2022.0257.
- Benko AL, McAloose CA, Becker PM, et al. Repository corticotrophin injection exerts direct acute effects on human B cell gene expression distinct from the actions of glucocorticoids. Clin Exp Immunol. 2018;192(1):68‐81.
- Gong R. The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol. 2011;8(2):122-128.
- Caruso V, Lagerström MC, Olszewski PK, Fredriksson R, Schiöth HB. Synaptic changes induced by melanocortin signalling. Nat Rev Neurosci. 2014;15(2):98‐110.
- Zhong Q, Sridhar S, Ruan L, et al. Multiple melanocortin receptors are expressed in bone cells. Bone. 2005;36(5):820‐831.
- Lisak RP, Benjamins JA. Melanocortins, melanocortin receptors and multiple sclerosis. Brain Sci. 2017;7(104):1‐18.
- Taylor AW, Lee D. Applications of the role of α‐MSH in ocular immune privilege. Adv Exp Med Biol. 2010;681:143‐149.
- Artuc M, Grützkau A, Luger T, Henz BM. Expression of MC1‐ and MC5‐receptors on the human mast cell line HMC‐1. Ann N Y Acad Sci. 1999;885:364‐367.
- Chang M, Chen B, Shaffner J, Dworkin LD, Gong R. Melanocortin system in kidney homeostasis and disease: novel therapeutic opportunities. Front Physiol. 2021;12:651236.
- Lisak R, Bealmear B, Nedlekoska L, et al. Schwann cells express melanocortin receptor subtypes: activation by ACTH 1–39 and alpha‐MSH enhances proliferation [abstract P1.430]. Neurology. 2018;90(suppl 15):1‐2.
- Kaneva MK, Kerrigan MJ, Grieco P, Curley GP, Locke IC, Getting SJ. Chondroprotective and anti‐inflammatory role of melanocortin peptides in TNF‐α activated human C‐20/A4 chondrocytes. Br J Pharmacol. 2012;167(1):67‐79.
- Grässel S, Opolka A, Anders S, et al. The melanocortin system in articular chondrocytes: melanocortin receptors, pro‐opiomelanocortin, precursor proteases, and a regulatory effect of alpha‐melanocyte‐stimulating hormone on proinflammatory cytokines and extracellular matrix components. Arthritis Rheum. 2009;60(10):3017‐3027.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Poola N, Due B, Wright D, Brooks LR, Zaman F. Pharmacokinetics and pharmacodynamics of repository corticotropin injection compared with synthetic ACTH1-24 depot and methylprednisolone in healthy subjects [published online ahead of print]. Clin Pharmacol Drug Dev. 2021. doi:10.1002/cpdd.1020.
- Data on file: REF-03003. Mallinckrodt ARD LLC.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Olsen NJ, Decker DA, Higgins P, et al. Direct effects of HP Acthar Gel on human B-lymphocyte activation in vitro. Arthritis Res Ther. 2015;17:300.
- Data on file: REF-04768. Mallinckrodt ARD LLC
- Healy LM, Jang JH, Lin YH, Rao V, Antel JP, Wright D. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages [ECTRIMS-ACTRIMS abstract EP14841]. Mult Scler J. 2017;23(suppl 3):777.
- Healy LM, Lin YH, Jang JH, Rao V, Antel JP, Wright D. Melanocortin receptor mediated anti-inflammatory effect of repository corticotropin injection on human monocyte-derived macrophages. Poster presented at: 7th Joint ECTRIMS-ACTRIMS Meeting; October 25-28, 2017; Paris, France. Poster EP1481.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Aggarwal R, Marder G, Koontz DC, Nandkumar P, Qi Z, Oddis CV. Efficacy and safety of adrenocorticotropic hormone gel in refractory dermatomyositis and polymyositis. Ann Rheum Dis. 2018;77(5):720-727.
- Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. 2014;23(9):905-912.
- Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327-344.
- Fiechtner JJ, Montroy T, June J. A single-site, investigator initiated open-label trial of H.P. Acthar® Gel (repository corticotropin injection) an adrenocorticotropic hormone (ACTH) analogue in subjects with moderately to severely active psoriatic arthritis (PsA). J Dermatol Res Ther. 2016;2(5):1-7.
- Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313-322.
- Zand L, Canetta P, Lafayette R, et al. An open-label pilot study of adrenocorticotrophic hormone in the treatment of IgA nephropathy at high risk of progression. Kidney Int Rep. 2020;5(1):58-65.
- Wirta D, McLaurin E, Ousler G, Liu J, Kacmaz RO, Grieco J. Repository corticotropin injection (Acthar® Gel) for refractory severe noninfectious keratitis: efficacy and safety from a phase 4, multicenter, open-label study. Ophthalmol Ther. 2021;10(4):1077-1092.
- Anesi SD, Chang PY, Maleki A, et al. Treatment of noninfectious retinal vasculitis using subcutaneous repository corticotropin injection. J Ophthalmic Vis Res. 2021;16(2):219-233.
- Bryan MS, Sergott RC. Changes in visual acuity and retinal structures following repository corticotropin injection (RCI) therapy in patients with acute demyelinating optic neuritis: improvement in low contrast visual acuity in both affected and contralateral eyes in a single-armed open-label study. J Neurol Sci. 2019;407:116505.
- Wynn D, Goldstick L, Bauer W, et al. Results from a multi-center, randomized, double-blind, placebo-controlled study of repository corticotropin injection for multiple sclerosis relapse that did not adequately respond to corticosteroids. CNS Neurosci Ther. 2022;28:364-371.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Acthar Gel (repository corticotropin injection) Single-Dose Pre-filled SelfJect Injector, 40 USP Units/0.5 mL [Instructions for Use]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Acthar Gel (repository corticotropin injection) Single-Dose Pre-filled SelfJect Injector, 80 USP Units/1.0 mL [Instructions for Use]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Data on File — Ref-07341. Mallinckrodt Pharmaceuticals.
References:
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Kaplan J, Askanase A, Chu D, et al. Acthar® Gel treatment for patients with autoimmune and inflammatory diseases: an historical perspective and characterization of clinical evidence. Clin Drug Invest. 2023;43:739-761.
- Saygin D, Oddis CV, Marder G, et al. Follow-up results of myositis patients treated with Acthar gel. Rheumatology (Oxford). 2020;59(10):2976-2981.
- Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327-344.
- Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13:1-11.
- Baughman RP, Barney JB, O'Hare L, Lower EE. A retrospective pilot study examining the use of Acthar Gel in sarcoidosis patients. Respir Med. 2016;110:66-72.
- Zand L, Canetta P, Lafayette R, et al. An open-label pilot study of adrenocorticotrophic hormone in the treatment of lgA nephropathy at high risk of progression. Kidney Int Rep. 2020;5(1):58·65.
- Alhamad T, Manllo Dieck J, Younus U, et al. ACTH gel in resistant focal segmental glomerulosclerosis after kidney transplantation. Transplantation. 2019;103(1):202-209.
- Nelson WW, Lima AF, Kranyak J, et al. Retrospective medical record review to describe use of repository corticotropin injection among patients with uveitis in the United States. J Ocul Pharmacol Ther. 2019;35(3):182-188.
- Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11:598496.
- Bryan MS, Sergott RC. Changes in visual acuity and retinal structures following repository corticotropin injection (RCI) therapy in patients with acute demyelinating optic neuritis: improvement in low contrast visual acuity in both affected and contralateral eyes in a single-armed open-label study. J Neurol Sci. 2019;407:116505.
- Aggarwal R, Marder G, Koontz DC, Nandkumar P, Qi Z, Oddis CV. Efficacy and safety of adrenocorticotropic hormone gel in refractory dermatomyositis and polymyositis. Ann Rheum Dis. 2018;77(5):720-727.
- Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313-322.
References:
- Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327-344.
- US Department of Health and Human Services. Enrichment strategies for clinical trials to support determination of effectiveness of human drugs and biological products. Guidance for industry. March 2019. https://www.fda.gov/media/121320/download. Accessed June 11, 2019.
- Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. A multicenter study assessing the efficacy and safety of repository corticotropin injection in patients with persistently active rheumatoid arthritis. Poster presented at: European Congress of Rheumatology; June 12-15, 2019; Madrid, Spain.
- Curtis JR, Yang S, Chen L, et al. Determining the minimally important difference in the clinical disease activity index for improvement and worsening in early rheumatoid arthritis patients. Arthritis Care Res (Hoboken). 2015;67(10):1345-1353.
- Orbai AM, Bingham CO III. Patient reported outcomes in rheumatoid arthritis clinical trials. Curr Rheumatol Rep. 2015;17(4):28.
References:
- Ho-Mahler N, Turner B, Eaddy M, Hanke ML, Nelson WW. Treatment with repository corticotropin injection in patients with rheumatoid arthritis, systemic lupus erythematosus, and dermatomyositis/polymyositis. Open Access Rheumatol. 2020;12:21-28.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
References:
- Aggarwal R, Marder G, Koontz DC, Nandkumar P, Qi Z, Oddis CV. Efficacy and safety of adrenocorticotropic hormone gel in refractory dermatomyositis and polymyositis. Ann Rheum Dis. 2018;77(5):720-727.
- National Institute of Environmental Health Sciences. Disease activity core set measures. https://www.niehs.nih.gov/research/resources/imacs/diseaseactivity/index.cfm. Updated August 31, 2017. Accessed October 10, 2022.
References:
- Saygin D, Oddis CV, Marder G, et al. Follow-up results of myositis patients treated with Acthar gel. Rheumatology (Oxford). 2020;59(10):2976-2981.
References:
- Fiechtner JJ, Montroy T. Treatment of moderately to severely active systemic lupus erythematosus with adrenocorticotropic hormone: a single-site, open-label trial. Lupus. 2014;23(9):905-912.
References:
- Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11:598496.doi:10.3389/fneur.2020.598496.
- Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald Criteria. Ann Neurol. 2011;69(2):292-302.
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(pt 5):962-973.
- Jones KH, Ford DV, Jones PA, et al. The physical and psychological impact of multiple sclerosis using the MSIS-29 via the web portal of the UK MS Register. PLoS One. 2013;8(1):e5542. doi:10.1371/journal.pone.0055422.
- Costelloe L, O'Rourke K, Kearney H, et al. The patient knows best: significant change in the physical component of the Multiple Sclerosis Impact Scale (MSIS-29 physical). J Neurol Neurosurg Psychiatry. 2007;78(8):841-844.
- Widener GL, Allen DD. Measurement characteristics and clinical utility of the 29-item Multiple Sclerosis Impact Scale. Arch Phys Med Rehabil. 2014;95(3):593-594.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452.
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
References:
- Bryan MS, Sergott RC. Changes in visual acuity and retinal structures following repository corticotropin injection (RCI) therapy in patients with acute demyelinating optic neuritis: improvement in low contrast visual acuity in both affected and contralateral eyes in a single-armed open-label study. J Neurol Sci. 2019;407:116505. doi:10.1016/j.jns.2019.116505.
References:
- Knupp KG, Coryell J, Nickels KC, et al. Response to treatment in a prospective national infantile spasms cohort. Ann Neurol. 2016;79(3):475-484.
References:
- Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, Khastgir A. Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17(1):37. doi:10.1186/s12882-016-0241-7.
- Data on File – Ref-00742. Mallinckrodt Pharmaceuticals.
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. Clinical practice guideline for glomerulonephritis. Kidney Int Suppl. 2012;2(2):139-274.
References:
- Baughman RP, Barney JB, O'Hare L, Lower EE. A retrospective pilot study examining the use of Acthar gel in sarcoidosis patients. Respir Med. 2016;110:66-72.
References:
- Baughman RP, Sweiss N, Keijsers R, et al. Repository corticotropin for chronic pulmonary sarcoidosis. Lung. 2017;195(3):313-322.
References:
- Data on File – Ref-05336. Mallinckrodt Pharmaceuticals.
- Data on File – Ref-05814. Mallinckrodt Pharmaceuticals.
- Fleischmann R, Furst DE, Connolly-Strong E, Liu J, Zhu J, Brasington R. Repository corticotropin injection for active rheumatoid arthritis despite aggressive treatment: a randomized controlled withdrawal trial. Rheumatol Ther. 2020;7(2):327-344.
- Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13:1753466619888127. doi:10.1177/1753466619888127.
- Wirta D, McLaurin E, Ousler G, Liu J, Kacmaz RO, Grieco J. Repository corticotropin injection (Acthar® Gel) for refractory severe noninfectious keratitis: efficacy and safety from a phase 4, multicenter, open-label study. Ophthalmol Ther. 2021;10(4):1077-1092.
- Zand L, Canetta P, Lafayette R, et al. An open-label pilot study of adrenocorticotrophic hormone in the treatment of IgA nephropathy at high risk of progression. Kidney Int Rep. 2020;5(1):58-65.
- Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11:598496.doi:10.3389/fneur.2020.598496.
References:
- Alhamad T, Manllo Dieck J, Younus U, et al. ACTH gel in resistant focal segmental glomerulosclerosis after kidney transplantation. Transplantation. 2019;103(1):202-209.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Hladunewich MA, Cattran D, Beck LH, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29(8):1570-1577.
- Bomback AS, Canetta PA, Beck LH Jr, Ayalon R, Radhakrishnan J, Appel GB. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36(1):58-67.
- Madan A, Mijovic-Das S, Stankovic A, Teehan G, Milward AS, Khastgir A. Acthar Gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17:37.
- Tumlin J, Galphin C, Santos R, Rovin B. Kidney Int Rep. 2017;2(5):924-932.
- Bomback AS, Tumlin JA, Baranski J, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Devel Ther. 2011;5:147-153.
- Filippone EJ, Dopson SJ, Rivers DM, et al. Adrenocorticotropic hormone analog use for podocytopathies. Int Med Case Rep J. 2016;9:125-133.
- Hogan J, Bomback AS, Mehta K, et al. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol. 2013;8(12):2072-2081.
References:
- Zand L, Canetta P, Lafayette R, et al. An open-label pilot study of adrenocorticotrophic hormone in the treatment of lgA nephropathy at high risk of progression. Kidney Int Rep. 2020;5(1):58-65.
References:
- Baram TZ, Mitchell WG, Tournay A, Snead OC, Hanson RA, Horton EJ. High-dose corticotropin (ACTH) versus prednisone for infantile spasms: a prospective, randomized, blinded study. Pediatrics. 1996;97(3):375-379.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
References:
- Data on file: REF-MNK14084113. Mallinckrodt ARD LLC.
References:
- Bryan MS, Sergott RC. Changes in visual acuity and retinal structures following repository corticotropin injection (RCI) therapy in patients with acute demyelinating optic neuritis: improvement in low contrast visual acuity in both affected and contralateral eyes in a single-armed open-label study. J Neurol Sci. 2019;407:116505. doi:10.1016/j.jns.2019.116505.
References:
- Fiechtner JJ, Montroy T, June J. A single-site, investigator initiated open-label trial of H.P. Acthar® Gel (repository corticotropin injection) an adrenocorticotropic hormone (ACTH) analogue in subjects with moderately to severely active psoriatic arthritis (PsA). J Dermatol Res Ther. 2016;2(5):1-7.
- Schmitt J, Wozel G. The psoriasis area and severity index is the adequate criterion to define severity in chronic plaque-type psoriasis. Dermatology. 2005;210(3):194-199.
References:
- Chopra I, Qin Y, Kranyak J, et al. Repository corticotropin injection in patients with advanced symptomatic sarcoidosis: retrospective analysis of medical records. Ther Adv Respir Dis. 2019;13:1753466619888127. doi:10.1177/1753466619888127.
References:
- Tumlin J, Galphin C, Santos R, Rovin B. Kidney Int Rep. 2017;2(5):924-932.
References:
- Nelson WW, Lima AF, Kranyak J, et al. Retrospective medical record review to describe use of repository corticotropin injection among patients with uveitis in the United States. J Ocul Pharmacol Ther. 2019;35(3):182-188.
References:
- Wirta D, McLaurin E, Ousler G, Liu J, Kacmaz RO, Grieco J. Repository corticotropin injection (Acthar® Gel) for refractory severe noninfectious keratitis: efficacy and safety from a phase 4, multicenter, open-label study. Ophthalmol Ther. 2021;10(4):1077-1092.
- Data on File – Ref-04624. Mallinckrodt Pharmaceuticals.
- US Food and Drug Administration Center for Drug Evaluation and Research. Clinical outcome assessment review. 2016. Accessed January 19, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208073Orig1s000MedR.pdf.
- Grubbs JR, Tolleson-Rinehart S, Huynh K, Davis RM. A review of quality of life measures in dry eye questionnaires. Cornea. 2014;33(2):215-218.
- Abetz L, Rajagopalan K, Mertzanis P, Begley C, Barnes R, Chalmers R; Impact of Dry Eye on Everyday Life (IDEEL) Study Group. Development and validation of the impact of dry eye on everyday life (IDEEL) questionnaire, a patient-reported outcomes (PRO) measure for the assessment of the burden of dry eye on patients. Health Qual Life Outcomes. 2011;9:111.
- Fairchild CJ, Chalmers RL, Begley CG. Clinically important difference in dry eye: change in IDEEL-symptom bother. Optom Vis Sci. 2008;85(8):699-707.
References:
- Rahaghi FF, Sweiss NJ, Saketkoo LA, et al. Management of repository corticotrophin injection therapy for pulmonary sarcoidosis: a Delphi study. Eur Respir Rev. 2020;29(155):190147. doi:10.1183/16000617.0147-2019.
- Baughman RP, Scholand MB, Rahaghi FF. Clinical phenotyping: role in treatment decisions in sarcoidosis. Eur Respir Rev. 2020;29(155):190145. doi:10.1183/16000617.0145-2019.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
- Rahaghi FF, Baughman RP, Saketkoo LA, et al. Delphi consensus recommendations for a treatment algorithm in pulmonary sarcoidosis. Eur Respir Rev. 2020;29(155):190146. doi:10.1183/16000617.0146-2019.
References:
- Data as of 01/22. Data on File – Ref-05336. Mallinckrodt Pharmaceuticals.
References:
- Data on File – Ref-05118. Mallinckrodt Pharmaceuticals.
References:
- Data on File – Ref-05292. Mallinckrodt Pharmaceuticals.
References:
- Data on File – Ref-05291. Mallinckrodt Pharmaceuticals.
References:
- Data on File – Ref-04568. Mallinckrodt Pharmaceuticals.
- CellCept (mycophenolate mofetil) [prescribing information]. San Francisco, CA: Genentech USA, Inc.
References:
- Data on File – Ref-04990. Mallinckrodt Pharmaceuticals.
References:
- Madan A. Repository corticotropin injection in a patient presenting with focal segmental glomerulosclerosis, rheumatoid arthritis, and optic neuritis: a case report. Int J Gen Med. 2015;8:119-124.
References:
- Fernandez AP, Gallop J, Polly S, Khanna U. Efficacy and safety of repository corticotropin injection for refractory cutaneous dermatomyositis: a prospective, open-label study. Rheumatology (Oxford). 2023 Nov 6. Epub ahead of print. doi: 10.1093/rheumatology/kead595
- Anyanwu CO, Fiorentino DF, Chung L, et al. Validation of the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI): characterizing disease severity and assessing responsiveness to clinical change. Br J Dermatol. 2015;173(4):969-974.
References:
- Anesi SD, Chang PY, Maleki A, et al. Treatment of noninfectious retinal vasculitis using subcutaneous repository corticotropin injection. J Ophthalmic Vis Res. 2021;16(2):219-233.
References:
- Anesi SD, Chang PY, Maleki A, et al. Effects of subcutaneous repository corticotropin gel injection on regulatory T cell population in noninfectious retinal vasculitis. Ocul Immunol Inflamm. 2023;31(3):556-565.
References:
- Hladunewich MA, Cattran D, Beck LH, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar® Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29(8):1570-1577.
- Acthar Gel (repository corticotropin injection) [prescribing information]. Bridgewater, NJ: Mallinckrodt ARD LLC.
References:
- Wynn D, Goldstick L, Bauer W, et al. Results from a multi-center, randomized, double-blind, placebo-controlled study of repository corticotropin injection for multiple sclerosis relapse that did not adequately respond to corticosteroids. CNS Neurosci Ther. 2022;28:364-371.
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33(11):1444-1452.
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28-37.
- Hobart J, Lamping D, Fitzpatrick R, Riazi A, Thompson A. The Multiple Sclerosis Impact Scale (MSIS-29): a new patient-based outcome measure. Brain. 2001;124(Pt 5):962-973.
- Kaplan J, Miller T, Baker M, Due B, Zhao E. A prospective observational registry of repository corticotropin injection (Acthar® Gel) for the treatment of multiple sclerosis relapse. Front Neurol. 2020;11:598496.
References:
- Data on File – Ref-07075. Mallinckrodt Pharmaceuticals.
References:
- Data on File – Ref-07195. Mallinckrodt Pharmaceuticals.